Quantitative PET-CT and SPECT-CT
Our Center is very active in several research areas pertaining to quantitative positron emission tomography, both in PET-CT and PET-MR. Topics range from absolute quantitation with novel scatter, attenuation, and randoms corrections in model-based iterative reconstructions both in PET-CT and PET-MR to quantitative imaging in non-traditional radioisotopes such as Y-86, and absolute quantitation of neurotransmitter densities in dynamic PET imaging.
Quantitative PET
Absolute Quantitation of Myocardial Blood Flow Using Dynamic PET
Absolute Quantitation of Myocardial Blood Flow Using Dynamic PET
The central hypothesis of this proposal is that objective quantitation of myocardial perfusion and coronary flow reserve can be achieved using 82Rb imaging and, furthermore, that these measures are important determinants of clinical risk and, thus, useful for optimizing management decisions in patients with coronary artery disease (CAD). We use dynamic 82Rb PET along with innovative approaches based on generalized factor analysis of dynamic sequences (GFADS), that allow automatic estimation of left and right ventricle input functions, as well as region-based compartment analysis to characterize and quantify the coronary flow reserve (CFR) as well as the severity and extent of perfusion abnormalities that occur in CAD.
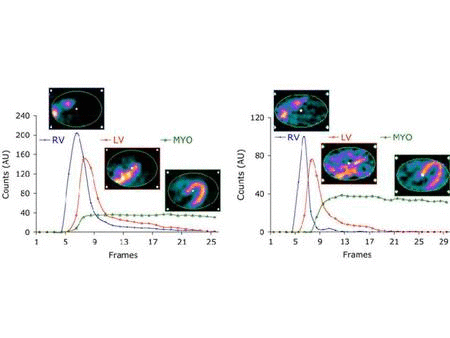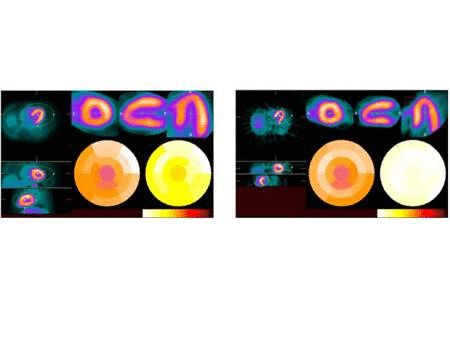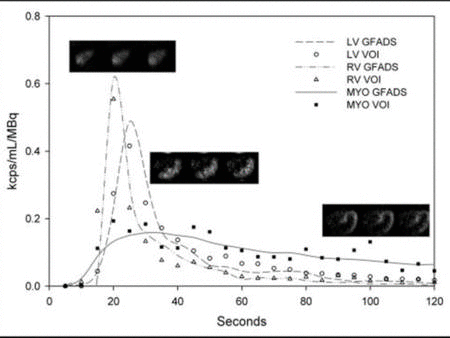
Related Papers:
- El Fakhri G., Kardan A., Sitek A., Dorbala S., Abi-Hatem N., Lahoud Y., Fischman A.J., Coughlan M., Yasuda T., Di Carli M.F. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment Using 82Rb-PET: Comparison with 13N-Ammonia. J. Nucl. Med. 2009; 50: 1062-1071.
- Anagnostopoulos C., Almonacid A., El Fakhri G., Currilova Z., Sitek A., Roughton M., Dorbala S., Popma J., Di Carli M. Quantitative Relationship Between Coronary Vasodilator Reserve Assessed by Rubidium-82 PET Imaging and Coronary Artery Stenosis Severity. Eur. J. Nucl. Med. Mol. Imag. 2008; 35: 1593-1601.
- Di Carli M.F., Dorbala S., Meserve J., El Fakhri G., Sitek A., Moore S.C. Clinical myocardial perfusion PET-CT. J. Nucl. Med; 2007; 48: 783-793.
- El Fakhri G., Sitek A., Guérin B., Kijewski M.F., Di Carli M.F., and Moore S.C. Quantitative dynamic cardiac 82Rb-PET imaging using generalized factor and compartment analyses. J. Nucl. Med. 2005; 46: 1264-1271 (2006 Mosby-Year Book of Nuclear Medicine).
Scatter Compensation in PET
Scatter Compensation in PET
The overall goal of this project is to develop improved techniques for quantitative PET imaging. Recently, we have reformulated the PET reconstruction problem in order to include the energy of detected photons, which is a measurement not currently used in commercial scanner but has the potential of improving significantly the accuracy of scatter correction. Specifically, this work involved working a new expression of the list-mode PET likelihood function containing the 2D (because there are 2 photons per coincidence) energy probability density functions (EPDFs) of primary and scatter coincidences. This formulation of the likelihood lead to a novel MLEM reconstruction algorithm incorporating position and energy dependent corrections. Finally, analytical formulas were derived for estimating these EPDFs based on a decomposition of energy spectra into primary and scatter components. Our results on simulations indicate that 1) the energy information can be used to scale the scatter sinogram to the measured data and is more accurate than the traditional “tails fitting” approach for large patients; 2) the inclusion of energy PDFs in the MLEM reconstruction improves significantly the accuracy of the scatter correction in term of bias and contrast in cold regions.
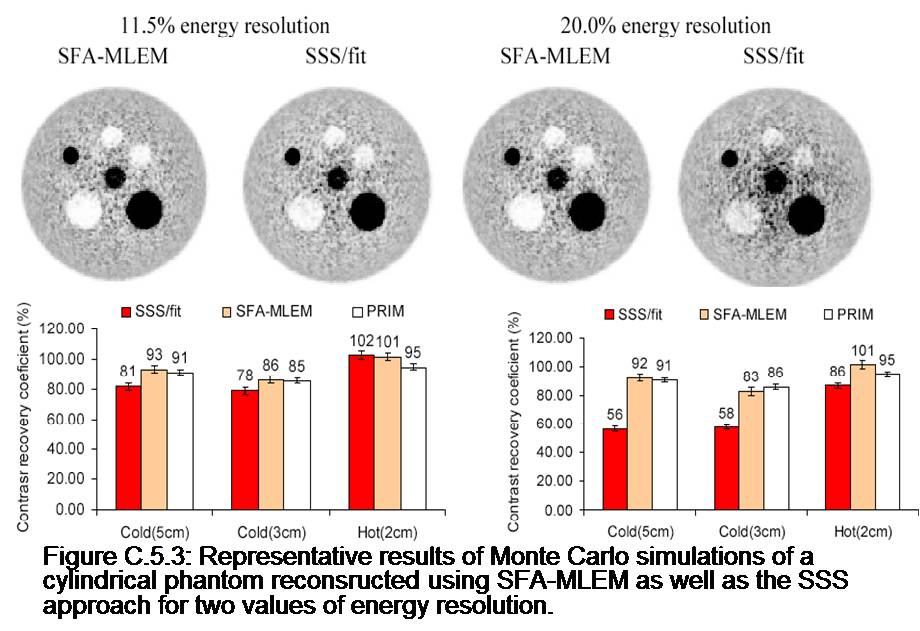
Related Papers:
Guerin B., Sitek A., and El Fakhri G., “Statistical energy-based scatter correction and reconstruction in list-mode PET”. J. Nucl. Med. 2008; 49.
Quantitative Y-86 PET for Personalized Radionuclide Therapy
Quantitative Y-86 PET for Personalized Radionuclide Therapy
Targeted radionuclide therapy (TRT) and radioimmunotherapy (RIT) are at the forefront of molecular cancer treatment modalities that involve the use of cancer cell-targeting radiopharmaceuticals, such as radiolabeled antibodies. Y-90 based therapy has thus far been hampered by the inability to accurately image and quantify the distribution of Y-90 within the body as the latter is a pure b emitter. Quantitative Y-86 PET imaging allows personalized patient treatment by enabling tailored Y-90 TRT based on the quantitative biodistribution of Y-86 uptake but presents unique challenges as it requires compensation for many physical effects including gamma cascade that greatly affect image quality and accuracy. We are accurately modeling the physics of PET imaging using isotopes with cascade gammas, and developing correction algorithms to achieve quantitative Y-86 PET dosimetry.
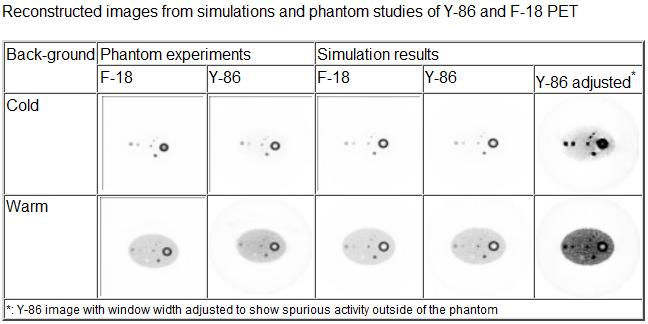
Related Papers:
- Zhu X.and El Fakhri G. Monte Carlo modeling of cascade gamma rays in 86Y PET imaging: Preliminary results. Phys. Med. Biol. 2009 Jul 7;54(13):4181-93.
Quantitative Imaging of Neurotransmitter and Receptor Systems
Quantitative Imaging of Neurotransmitter and Receptor Systems
In recent years a number of positron-emitting ligands have been developed, allowing study of neurotransmission and reception in the brain. The methodology for assessing quantities, such as receptor for density and occupancy, in vivo are based on prior in vitro assays. However, the in vivo situation is more complex, owing to the necessity to account for blood flow, blood-to-tissue transport mechanisms, and the dynamics of nonspecific binding of the ligands. Several different assays have been developed, ranging from single injection determinations of binding potential, to the use of multiple injections at different specific activities to determine absolute receptor density. A novel line of investigation for future research has been described along with analytic techniques – that use cognitive activation to modulate receptor occupancy.
Iterative Brain and Cardiac Image Reconstruction
By using the iterative image reconstruction one may obtain PET images with better quality. Our projects are focused on using a priori information in the iterative reconstruction to improve image quality and assist computation of the parametric images.

Application of Image Processing to Radiology
Application of Image Processing to Radiology
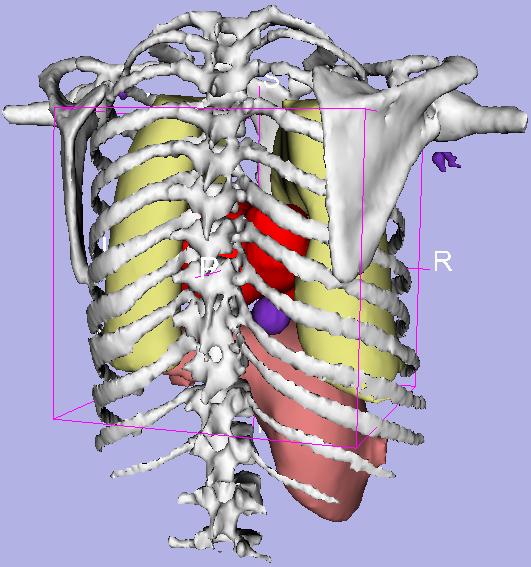
Research in this area has focused on development of algorithms for multi-modality registration of human and animal brain images and development of algorithms and techniques for parametric imaging of physiological processes. Image registration techniques allow image volumes taken under different circumstances (e.g. different times, different modalities, even different subjects) to be aligned (and possibly deformed) so that they can exactly overlay one another. This process is important for research and diagnosis when serial quantitative comparisons are necessary. Parametric images are formed by analyzing the concentration history of every voxel in PET data sets. Kinetic parameters for each voxel are presented as images, making it possible to provide a quantitative visualization of physiological process.
Direct Reconstruction of PET Kinetic Parameters
Direct Reconstruction of PET Kinetic Parameters
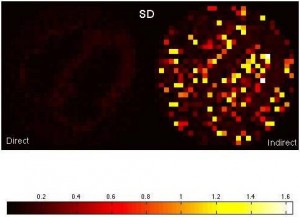
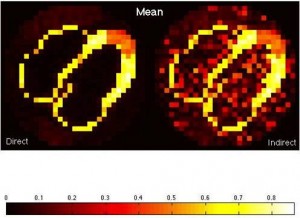
We are interested in an alternative way to estimate kinetic parameters in dynamic PET imaging. In this approach, we directly reconstruct PET parametric images from sinograms as compared to the conventional indirect approach where frame-by-frame images are reconstructed first and then followed by fitting time-activity curves voxel-wise. This direct approach has potential to reduce noise in parametric images because it does not impose assumptions about noise modeling and uncorrelatedness among voxels in the reconstructed images. To this end, we have developed a gradient-based direct reconstruction approach with improved convergence, and have assessed its performance in cardiac PET imaging.
Figure: Comparison of the mean and standard deviation images between the direct and indirect approaches in estimating K1.
Non-Local Means Denoising of Dynamic PET Images
Non-Local Means Denoising of Dynamic PET Images
Dutta J, Leahy RM, Li Q. Non-Local Means Denoising of Dynamic PET Images. PLoS One. 2013; 8(12):e81390.
Dynamic positron emission tomography (PET), which reveals information about both the spatial distribution and temporal kinetics of a radiotracer, enables quantitative interpretation of PET data. Model-based interpretation of dynamic PET images by means of parametric fitting, however, is often a challenging task due to high levels of noise, thus necessitating a denoising step. The objective of this paper is to develop and characterize a denoising framework for dynamic PET based on non-local means (NLM).
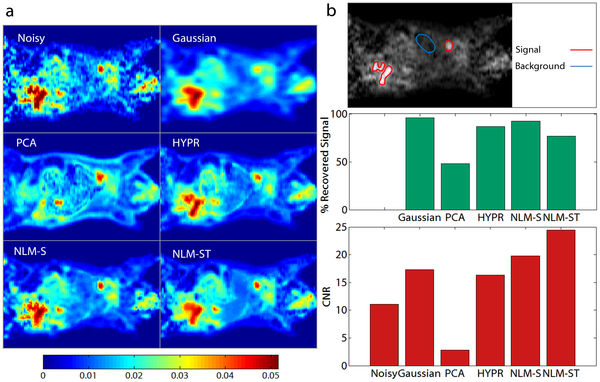
The overall objective of this work is to develop an NLM-based denoising framework for dynamic PET images and to assess the quantitative and qualitative merits of the resultant approach relative to other well-known image denoising approaches. We compare this technique with Gaussian denoising and principal component analysis (PCA) based denoising, both widely used in the context of dynamic PET imaging. In addition, we compare this technique with HighlY constrained backPRojection (HYPR) and conventional NLM denoising based on spatial patches. We perform a realistic simulation study based on a dynamic digital mouse phantom and compare the denoising methods by examining bias-variance characteristics of the denoised dynamic images and the corresponding Patlak parametric images. We then apply the developed method to denoise a preclinical 18F PET dataset from a mouse study and a clinical 18F PET dataset from a patient with hepatocellular carcinoma and perform Patlak analysis on these datasets.
Quantitative SPECT
Quantitative SPECT Imaging
Quantitative SPECT Imaging
We have developed compensation methods for Compton scatter, attenuation, variable collimator response as well as partial volume effect and septal penetration for many radionuclides used in SPECT such as Tc-99m, I-123, In-111, Ga-67, etc. Our methods range from spectral and dynamic factor analyses to fast and Monte Carlo-based iterative reconstruction and were applied to single and dual isotope SPECT in the brain, heart and cancer. Our lab has been one of the first to report successful imaging of simultaneous dual Tc-99m and I-123 in the brain and the heart in humans. We have also developed high-sensitivity centrally peaked collimators for a dedicated brain scanner and shown that a 3 fold gain in sensitivity can be achieved compared to triple head SPECT cameras. This collimator was manufactured and used in human brain studies. Finally, we have been heavily involved in the assessment of image quality in SPECT and the task-based improvement that can be achieved with these compensation methods that we have developed for lesion detection and activity quantitation.
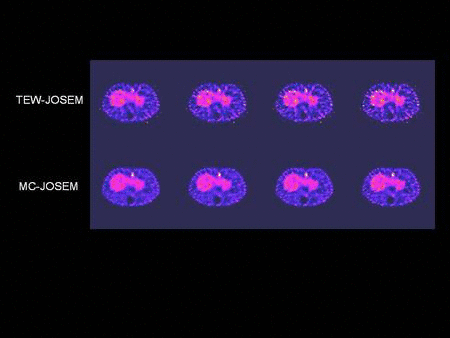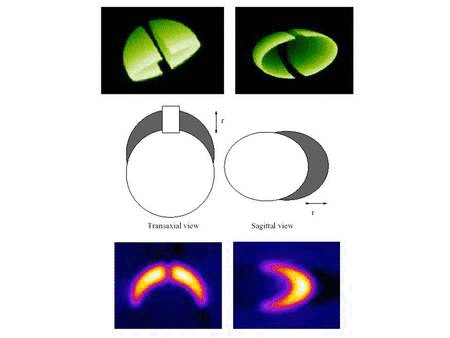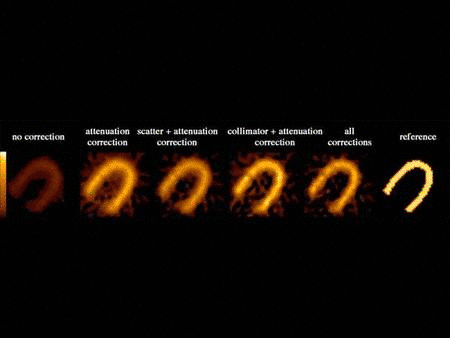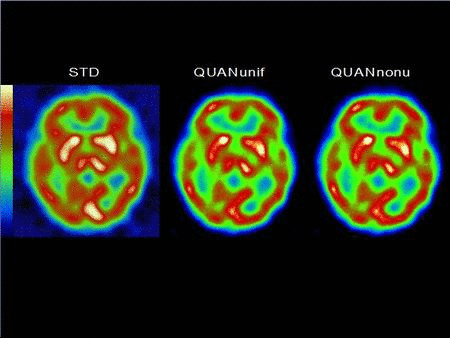
Related Papers:
- Ouyang J., El Fakhri G., Moore S.C. Improved activity estimation with MC-JOSEM versus TEW-JOSEM in 111In SPECT. Med. Phys. 2008; 35: 2029-2040.
- Zaidi H. and El Fakhri G. “Is absolute quantification of dopaminergic neurotransmission studies with 123I SPECT ready for clinical use?”. Eur. J. Nucl. Med. Mol. Imag. 2008; 35: 1330-1333.
- Mamede M., El Fakhri G., Abreu-e-Lima P., Gandler W., Nose V., Gerbaudo V. Pre-operative estimation of esophageal tumor metabolic length in FDG PET images with surgical pathology confirmation. Ann Nucl Med. 2007; 21: 553-562.
- El Fakhri G., Ouyang J., Zimmerman R.E., Fischman A.J., Kijewski M.F. Performance of a Novel Collimator for High–Sensitivity Brain SPECT. Med. Phys. 2006; 33:209-215.
- El Fakhri G., Sitek A., Zimmerman R.E., Ouyang J. Generalized Five Dimensional Dynamic and Spectral Factor Analysis. Med. Phys. 2006; 33: 1016-1024.
- Moore S.C., Ouyang J., Park M., El Fakhri G. Monte Carlo-based compensation for patient scatter, detector scatter, and crosstalk contamination in In-111 SPECT imaging. Nucl. Instrum. Meth. A. 2006; 569: 472-476.
- Ouyang J., El Fakhri G., Xia W., Kijewski M.F., Genna S. The Design and Manufacture of an Annular Variable-focusing Collimator for High-sensitivity Brain SPECT. IEEE Trans Nucl Sci 2006; 53: 2613-2618.
- Moore S.C., Kijewski M.F., and El Fakhri G. Collimator optimization for detection and quantitation tasks: application to gallium-67 imaging. IEEE Trans. Med. Imag; 2005; 24: 1347-1356.
- El Fakhri G., Kijewski M.F., Albert M.S., Johnson K.A., and Moore S.C. Quantitative SPECT leads to improved performance in discrimination tasks related to prodromal Alzheimer’s disease. J. Nucl. Med. 2004; 45: 2026-2031.
- El Fakhri G., Kijewski M.F., Johnson K.A., Syrkin G, Killiany R.J., Becker JA, Zimmerman R.E., Albert M.S. MRI-Guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer’s disease. Arch Neurol 2003; 60: 1066-1072.
- El Fakhri G., Moore S.C., Maksud P, and Kijewski M.F. The effects of compensation for scatter, lead x-rays and high-energy contamination on lesion detectability and activity estimation in Ga-67 imaging. IEEE Trans Nucl Sci 2003; 50: 439-445.
- El Fakhri G., Moore S.C., and Kijewski M.F. Optimization of Ga-67 imaging for detection and estimation tasks: dependence of imaging performance on spectral acquisition parameters. Med Phys 2002; 29: 1859-1866.
- El Fakhri G, Moore S.C., Maksud P. A new scatter compensation method for Ga-67 Imaging using artificial neural networks. IEEE Trans Nucl Sci.
- El Fakhri G, Kijewski M.F, Moore S.C. Absolute activity quantitation from projections using an analytical approach: comparison with iterative methods in brain SPECT. IEEE Trans Nucl Sci.
- El Fakhri G, Buvat I, Benali H, Todd-Pokropek A, Di Paola R. Relative impact of scatter, attenuation, collimator response and finite spatial resolution corrections in cardiac SPECT. J Nucl Med 2000;41:1400-1408.
- El Fakhri G, Maksud P, Kijewski M.F, Habert M.O, Todd-Pokropek A, Aurengo A, Moore S.C. Scatter and Cross-Talk Corrections in Simultaneous Tc-99m/I-123 Brain SPECT using Constrained Factor Analysis and Artificial Neural Networks. IEEE Trans Nucl Sci 2000;47:1573-1580.
- El Fakhri G, Buvat I, Almeida P, Bendriem B, Todd-Pokropek A, Benali H. Should scatter be corrected in both transmission and emission data for accurate quantitation in cardiac SPECT? Eur J Nucl Med 2000;27:1356-1364.
- Pélégrini M, Buvat I, El Fakhri G, Benali H, Grangeat P, Di Paola R. A spline-regularized minimal residual algorithm for iterative attenuation correction in SPECT. Phys Med Biol 1999; 10: 2623-2642.
- El Fakhri G, Buvat I, Pélégrini M, Benali H, Almeida P, Bendriem B, Todd-Pokropek A, Di Paola R. Respective roles of scatter, attenuation, collimator response and partial volume effect in cardiac SPECT quantitation: a Monte Carlo study. Eur J Nucl Med 1999 ; 26 : 437-446.
- El Fakhri G, Maksud P, Aurengo A. Evaluation of scatter correction methods using Monte Carlo simulation in non uniform media. SPIE 1998; 3338: 363-369.
- Maksud P, Fertil B, Rica C, El Fakhri G, Aurengo A. Artificial neural network as a tool to compensate for scatter and attenuation in radionuclide imaging. J Nucl Med 1998; 39; 4; 735-745.
- Pélégrini M, Benali H, Buvat I, El Fakhri G, Di Paola R. Two-dimensional statistical model for regularized backprojection in SPECT. Phys Med Biol 1998; 43: 421-434.
Factor Analysis of Medical Images
Factor Analysis of Medical Images
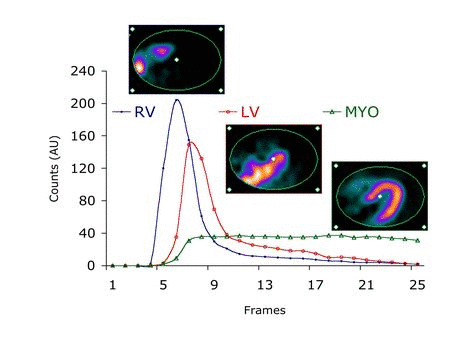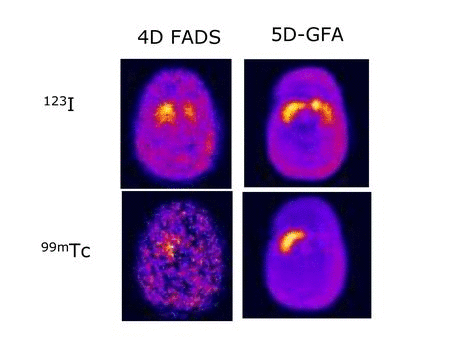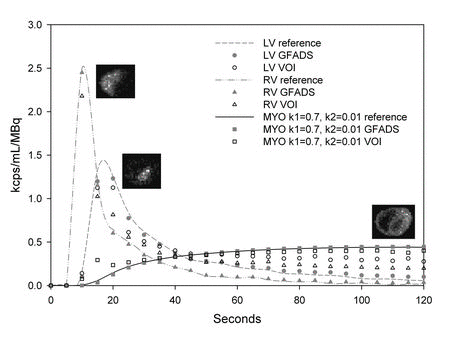
An active area of research in our group pertains to factor analysis of spectral (energy) and/or dynamic (time) sequences. Factor analysis is a powerful technique that allows the decomposition of a spectral or temporal image sequence into a small number of fundamental functions (factors) whose associated spatial distributions are called factor images. This, in turn, yields a synthetic representation of the contents of a relatively large dynamic or spectral image dataset. The major drawback of factor analysis, which precludes quantitation, is the non-uniqueness of the solution and we have developed several approaches to solve the non-uniqueness problem in brain and cardiac imaging.
Related Papers:
- El Fakhri G., Buvat I, Benali H, Todd-Pokropek A, Di Paola R. Relative Impact of Scatter, Collimator Response, Attenuation, and Finite Spatial Resolution Corrections in Cardiac SPECT. J Nucl Med 2000 ; 41 : 1400-1408 (published in the 2001 Mosby-Year Book of Nuclear Medicine).
- El Fakhri G., Maksud P, Kijewski M.F, Habert M.O, Todd-Pokropek A, Aurengo A, Moore S.C. Scatter and Cross-Talk Corrections in Simultaneous Tc-99m/I-123 Brain SPECT using Constrained Factor Analysis and Artificial Neural Networks. IEEE Trans Nucl Sci 2000 ; 47 :1573-1580.
- El Fakhri G., Maksud P., Kijewski M.F., et al. Quantitative simultaneous Tc-99m/I-123 SPECT: design study and validation with Monte Carlo simulations and physical acquisitions. IEEE Trans Nucl Sci 2002 ; 49: 2315-2321.
- El Fakhri G., Sitek A., Guérin B., Kijewski M.F., Di Carli M.F., and Moore S.C. Quantitative dynamic cardiac 82Rb-PET imaging using generalized factor and compartment analyses. J. Nucl. Med. 2005; 46: 1264-1271 (2006 Mosby-Year Book of Nuclear Medicine).
- El Fakhri G., Sitek A., Zimmerman R.E., Ouyang J. Generalized Five-Dimensional Dynamic and Spectral Factor Analysis. Med. Phys. 2006; 33: 1016-1024.
- Anagnostopoulos C., Almonacid A., El Fakhri G., Currilova Z., Sitek A., Roughton M., Dorbala S., Popma J., Di Carli M. Quantitative Relationship Between Coronary Vasodilator Reserve Assessed by Rubidium-82 PET Imaging and Coronary Artery Stenosis Severity. Eur. J. Nucl. Med. Mol. Imag. 2008; 35: 1593-1601.
- El Fakhri G., Kardan A., Sitek A., Dorbala S., Abi-Hatem N., Lahoud Y., Fischman A.J., Coughlan M., Yasuda T., Di Carli M.F. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment Using 82Rb-PET: Comparison with 13N-Ammonia. J. Nucl. Med. 2009; 50: 1062-1071.
Simultaneous Dual Tracer ECT
Simultaneous Dual Tracer ECT
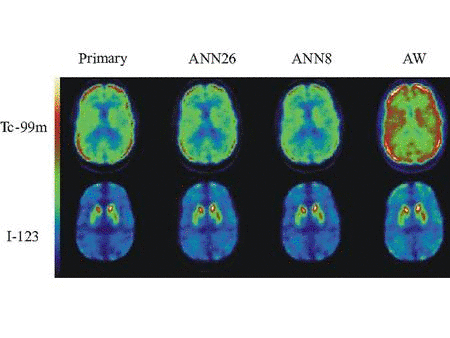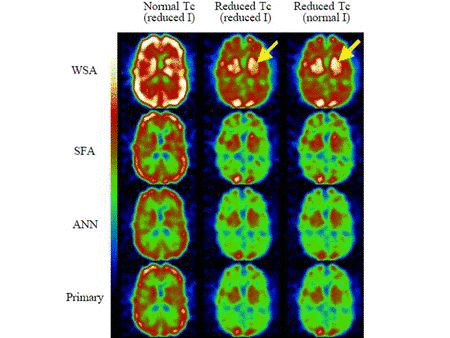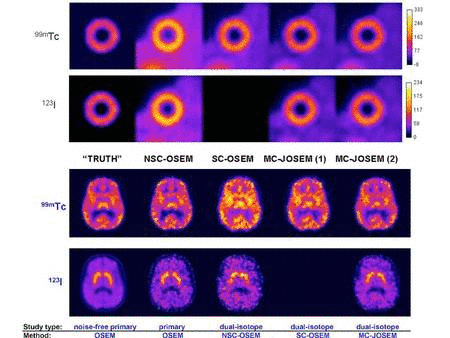
We are bringing to fruition our successful developments of accurate
compensation methods for physical factors affecting image quality and
reconstruction approaches for quantitative dual-isotope SPECT, and are expanding our scope to PET. We are presently evaluating the performance of two novel scatter correction methods for quantitative single and dual-isotope PET that we have developed. We are also extending our previous work in SPECT to novel approaches to dual-isotope dynamic brain PET using spatio-temporal information. We are assessing the performance of quantitative dual-isotope SPECT and PET in activity estimation tasks related to early diagnosis and quantitation of disease extent in coronary artery disease (CAD) and early Parkinson disease (PD) with or without dementia.
Dual-tracer PET using generalized factor analysis of dynamic sequences
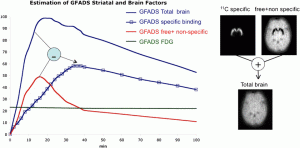
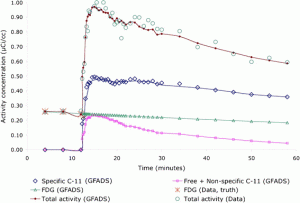
We developed a novel approach based on generalized factor analysis of dynamic sequences (GFADS) that exploits spatio-temporal differences between radiotracers and applied it to near-simultaneous imaging of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) (brain metabolism) and 11C-raclopride (D2) with simulated human data and experimental rhesus monkey data. We show theoretically and verify by simulation and measurement that GFADS can separate FDG and raclopride measurements that are made nearly simultaneously.
Estimation of specific and free + nonspecific 11C-raclopride. (Left) Time activity curves are calculated by subtraction of the (free + nonspecific) factor from the total brain factor after they have been scaled such that the factor images have the same mean values. (Right) (free + nonspecific) factor image is calculated by adding the spatially opposed factor images after they have been scaled such that their addition produces a uniform image. Resultant time activity factors for FDG and specifically and free + nonspecifically bound 11C-raclopride in a rhesus monkey study. Results were obtained by applying GFADS to a mid-striatal brain volume. Ground truth FDG activity is also shown for the initial three time frames.
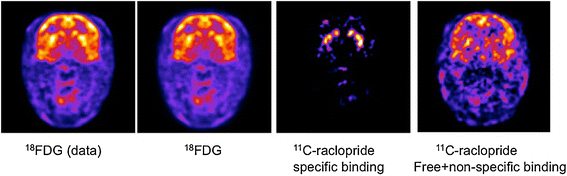
Factor images from application of GFADS to a mid-striatal, whole-brain slice of a dual-tracer rhesus monkey study. (Left to right) Ground truth FDG image (data), computed as the average of the initial three time frames; GFADS FDG image, demonstrating the same features as the ground truth image; GFADS specifically bound 11C-raclopride factor; derived free + nonspecifically bound GFADS factor image.
With kind permission from Springer Science+Business Media: Molecular Imaging and Biology, Dual-Tracer PET Using Generalized Factor Analysis of Dynamic Sequences, Volume 15, Issue 6, 2013, pp 666-674, Georges El Fakhri, Cathryn M. Trott, Arkadiusz Sitek, Ali Bonab, Nathaniel M. Alpert, Figures 1, 5, and 6
Related Papers:
- Trott C. and El Fakhri G. “Sequential and simultaneous dual-isotope brain SPECT: comparison with PET, for estimation and discrimination tasks in early parkinson disease”. Med. Phys., 2008; 35: 3343-3353.
- Ouyang J., Zhu X., Trott C., and El Fakhri G. Quantitative simultaneous 99mTc/123I cardiac SPECT using MC-JOSEM,” Med. Phys. 36, 602-611 (2009).
- Ouyang J., El Fakhri G., Moore S.C. Fast Monte Carlo Simulation Based Joint Iterative Reconstruction for Simultaneous 99mTc/123I Brain SPECT Imaging. Med. Phys. 2007; 34:x 3263-3272.
- El Fakhri G., Habert M.O., Maksud P., Kas A., Malek Z., Kijewski M.F., and Lacomblez L.. Quantitative simultaneous 99mTc-ECD/123I-FP-CIT SPECT in Parkinson disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imag. 2006; 33: 87-92.
- El Fakhri G., Maksud P., Kijewski M.F., et al. Quantitative simultaneous 99mTc/123I SPECT: design study and validation with Monte Carlo simulations and physical acquisitions. IEEE Trans Nucl Sci 2002 ; 49: 2315-2321.
- El Fakhri G, Moore S.C, Maksud P, Aurengo A, Kijewski M.F. Absolute activity quantitation in simultaneous I-123/Tc-99m brain SPECT. J Nucl Med 2001; 42: 300-308.
- El Fakhri G, Maksud P, Kijewski M.F, Habert M.O, Todd-Pokropek A, Aurengo A, Moore S.C. Scatter and Cross-Talk Corrections in Simultaneous Tc-99m/I-123 Brain SPECT using Constrained Factor Analysis and Artificial Neural Networks. IEEE Trans Nucl Sci 2000;47:1573-1580.