
Dr. Nappi presented her work at the Cardiovascular Council Young Investigator Award Symposium at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2018 Annual meeting in Philadelphia, and won the second place.

Dr. Nappi presented her work at the Cardiovascular Council Young Investigator Award Symposium at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2018 Annual meeting in Philadelphia, and won the second place.

Dr. Georges El Fakhri was elected Fellow to the Society of Nuclear Medicine and Molecular Imaging during a special plenary session at the society’s 2018 Annual Meeting, held June 23-26 in Philadelphia for his contributions to quantitative PET/CT/MR imaging. The SNMMI Fellowship was established in 2016 to recognize distinguished service to the society as well […]

Dr. Alexandre Detappe discusses his efforts to develop a novel imaging biomarker for early detection of Multiple Myeloma, a cancer of plasma cells.
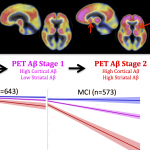
“PET staging of amyloidosis using striatum”: A new study published in Alzheimer’s and Dementia, the Journal of the Alzheimer’s Association, proposes to stage amyloid PET images using regional information. The research was conducted by Dr. Bernard Hanseeuw, instructor at MGH Gordon Center, and Dr. Keith Johnson, leader of the Aging NeuroImaging Program at the MGH Gordon Center, and the Harvard Aging Brain Study.

Dr. Aaron Schultz, PhD, Neurology, MGH/HMS presented his work on “Molecular Pathology in Aging and AD” at a recent Gordon Center Lecture

Dr. Lecomte was the guest speaker at a lecture on High Resolution PET Imaging from Mouse to Human Brain, organized by the MGH Gordon Center.

Dr. Peter Conti is a Professor at the University of Southern California with academic appointments in the Departments of Radiology, Biomedical Engineering, and Pharmaceutical Sciences. He received his medical degree from Cornell University and his Ph.D. in Biophysics from Memorial Sloan-Kettering Cancer Center. He is board certified in Nuclear Medicine (ABNM) and Diagnostic Radiology (ABR). […]